What Family Is Titanium in on the Periodic Table
 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Titanium | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pronunciation |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Advent | silvery greyness-white metal | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Standard atomic weight A r, std(Ti) | 47.867(1) [ii] | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Titanium in the periodic table | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Atomic number (Z) | 22 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Group | group 4 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Period | menstruum 4 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Block | d-cake | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Electron configuration | [Ar] 3d2 4sii | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Electrons per beat out | ii, 8, ten, 2 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Physical backdrop | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Phase atSTP | solid | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Melting indicate | 1941 K (1668 °C, 3034 °F) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Boiling bespeak | 3560 Yard (3287 °C, 5949 °F) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Density (nearr.t.) | 4.506 g/cmthree | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| when liquid (atm.p.) | 4.eleven one thousand/cm3 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Estrus of fusion | 14.xv kJ/mol | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Rut of vaporization | 425 kJ/mol | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Tooth heat chapters | 25.060 J/(mol·K) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Vapor pressure level
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Diminutive backdrop | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Oxidation states | −ii, −1, 0,[3] +one, +2 , +3 , +4 [4] (an amphoteric oxide) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Electronegativity | Pauling scale: 1.54 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Ionization energies |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Atomic radius | empirical: 147 pm | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Covalent radius | 160±viii pm | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Other properties | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Natural occurrence | primordial | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Crystal construction | hexagonal close-packed (hcp) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Speed of sound sparse rod | 5090 grand/s (atr.t.) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Thermal expansion | 8.vi µm/(thou⋅K) (at 25 °C) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Thermal conductivity | 21.9 W/(m⋅1000) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Electrical resistivity | 420 nΩ⋅m (at xx °C) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Magnetic ordering | paramagnetic | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Tooth magnetic susceptibility | +153.0×10−6 cm3/mol (293 Thou)[v] | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Immature'south modulus | 116 GPa | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Shear modulus | 44 GPa | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Majority modulus | 110 GPa | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Poisson ratio | 0.32 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Mohs hardness | half dozen.0 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Vickers hardness | 830–3420 MPa | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Brinell hardness | 716–2770 MPa | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| CAS Number | 7440-32-half dozen | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| History | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Discovery | William Gregor (1791) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| First isolation | Jöns Jakob Berzelius (1825) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Named by | Martin Heinrich Klaproth (1795) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Main isotopes of titanium | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Titanium is a chemical element with the symbol Ti and diminutive number 22. It is a lustrous transition element with a silver color, low density, and high strength, resistant to corrosion in body of water h2o, aqua regia, and chlorine.
Titanium was discovered in Cornwall, Slap-up Britain, past William Gregor in 1791 and was named past Martin Heinrich Klaproth subsequently the Titans of Greek mythology. The element occurs inside a number of mineral deposits, principally rutile and ilmenite, which are widely distributed in the Earth's chaff and lithosphere; information technology is establish in almost all living things, too equally bodies of water, rocks, and soils.[6] The metallic is extracted from its main mineral ores by the Kroll[vii] and Hunter processes. The most common chemical compound, titanium dioxide, is a popular photocatalyst and is used in the manufacture of white pigments.[8] Other compounds include titanium tetrachloride (TiClfour), a component of fume screens and catalysts; and titanium trichloride (TiClthree), which is used as a goad in the production of polypropylene.[half-dozen]
Titanium tin can exist alloyed with iron, aluminium, vanadium, and molybdenum, amid other elements, to produce strong, lightweight alloys for aerospace (jet engines, missiles, and spacecraft), armed services, industrial processes (chemicals and petrochemicals, desalination plants, pulp, and paper), automotive, agriculture (farming), medical prostheses, orthopedic implants, dental and endodontic instruments and files, dental implants, sporting goods, jewelry, mobile phones, and other applications.[6]
The 2 most useful properties of the metal are corrosion resistance and force-to-density ratio, the highest of any metallic element.[9] In its unalloyed condition, titanium is every bit strong as some steels, just less dense.[x] In that location are 2 allotropic forms[11] and v naturally occurring isotopes of this element, 46Ti through fiftyTi, with 48Ti being the virtually arable (73.viii%).[12] Although titanium and zirconium have the same number of valence electrons and are in the aforementioned group in the periodic table, they differ in many chemical and physical properties.
Characteristics
Concrete properties
As a metal, titanium is recognized for its high strength-to-weight ratio.[eleven] It is a stiff metal with depression density that is quite ductile (especially in an oxygen-free environment),[6] lustrous, and metallic-white in color.[13] The relatively high melting signal (1,668 °C or 3,034 °F) makes it useful every bit a refractory metal. Information technology is paramagnetic and has fairly low electric and thermal electrical conductivity compared to other metals.[6] Titanium is superconducting when cooled below its critical temperature of 0.49 K.[14] [fifteen]
Commercially pure (99.2% pure) grades of titanium have ultimate tensile strength of most 434 MPa (63,000 psi), equal to that of mutual, depression-class steel alloys, but are less dense. Titanium is 60% denser than aluminium, but more than twice every bit strong[ten] as the most commonly used 6061-T6 aluminium blend. Certain titanium alloys (due east.m., Beta C) reach tensile strengths of over 1,400 MPa (200,000 psi).[16] Yet, titanium loses strength when heated above 430 °C (806 °F).[17]
Titanium is not as difficult as some grades of estrus-treated steel; it is non-magnetic and a poor usher of estrus and electricity. Machining requires precautions, considering the material tin gall unless sharp tools and proper cooling methods are used. Similar steel structures, those fabricated from titanium have a fatigue limit that guarantees longevity in some applications.[xiii]
The metallic is a dimorphic allotrope of an hexagonal α form that changes into a trunk-centered cubic (lattice) β form at 882 °C (i,620 °F).[17] The specific oestrus of the α form increases dramatically as it is heated to this transition temperature but and then falls and remains adequately constant for the β form regardless of temperature.[17]
Chemical properties
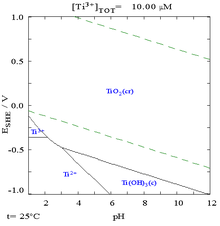
Like aluminium and magnesium, the surface of titanium metal and its alloys oxidize immediately upon exposure to air to class a thin not-porous passivation layer that protects the bulk metal from further oxidation or corrosion.[6] When it first forms, this protective layer is only 1–2 nm thick but information technology continues to grow slowly, reaching a thickness of 25 nm in four years.[nineteen] This layer gives titanium excellent resistance to corrosion, almost equivalent to platinum.
Titanium is capable of withstanding attack past dilute sulfuric and hydrochloric acids, chloride solutions, and virtually organic acids.[vii] However, titanium is corroded by concentrated acids.[xx] Equally indicated by its negative redox potential, titanium is thermodynamically a very reactive metal that burns in normal atmosphere at lower temperatures than the melting point. Melting is possible only in an inert atmosphere or in a vacuum. At 550 °C (1,022 °F), it combines with chlorine.[7] Information technology also reacts with the other halogens and absorbs hydrogen.[8]
Titanium readily reacts with oxygen at i,200 °C (2,190 °F) in air, and at 610 °C (1,130 °F) in pure oxygen, forming titanium dioxide.[11] Titanium is one of the few elements that burns in pure nitrogen gas, reacting at 800 °C (ane,470 °F) to grade titanium nitride, which causes embrittlement.[21] Considering of its high reactivity with oxygen, nitrogen, and many other gases, titanium that is evaporated from filaments is the basis for titanium sublimation pumps, in which titanium serves as a scavenger for these gases by chemically bounden to them. Such pumps inexpensively produce extremely low pressures in ultra-high vacuum systems.
Occurrence
Titanium is the ninth-almost abundant element in Earth's crust (0.63% by mass)[22] and the seventh-most arable metal. It is present every bit oxides in almost igneous rocks, in sediments derived from them, in living things, and natural bodies of water.[6] [seven] Of the 801 types of igneous rocks analyzed by the United States Geological Survey, 784 contained titanium. Its proportion in soils is approximately 0.5 to 1.five%.[22]
Common titanium-containing minerals are anatase, brookite, ilmenite, perovskite, rutile, and titanite (sphene).[19] Akaogiite is an extremely rare mineral consisting of titanium dioxide. Of these minerals, merely rutile and ilmenite have economic importance, withal even they are difficult to find in high concentrations. Nigh 6.0 and 0.7 million tonnes of those minerals were mined in 2011, respectively.[23] Pregnant titanium-bearing ilmenite deposits exist in western Commonwealth of australia, Canada, Cathay, India, Mozambique, New Zealand, Norway, Sierra Leone, South Africa, and Ukraine.[19] About 210,000 tonnes of titanium metal sponge were produced in 2020, mostly in Cathay (110,000 t), Nippon (50,000 t), Russia (33,000 t) and Kazakhstan (15,000 t). Total reserves of anatase, ilmenite, and rutile are estimated to exceed 2 billion tonnes.[23]
| Country | chiliad tonnes | % of total |
|---|---|---|
| China | iii,830 | 33.1 |
| Australia | 1,513 | 13.1 |
| Mozambique | 1,070 | 9.iii |
| Canada | 1,030 | 8.9 |
| Due south Africa | 743 | six.4 |
| Republic of kenya | 562 | 4.9 |
| India | 510 | 4.4 |
| Senegal | 502 | 4.3 |
| Ukraine | 492 | four.3 |
| World | eleven,563 | 100 |
The concentration of titanium is about 4 picomolar in the ocean. At 100 °C, the concentration of titanium in water is estimated to exist less than 10−7 M at pH seven. The identity of titanium species in aqueous solution remains unknown because of its depression solubility and the lack of sensitive spectroscopic methods, although only the four+ oxidation land is stable in air. No show exists for a biological role, although rare organisms are known to accrue high concentrations of titanium.[24]
Titanium is independent in meteorites, and it has been detected in the Lord's day and in K-type stars[seven] (the coolest blazon) with a surface temperature of iii,200 °C (5,790 °F).[25] Rocks brought back from the Moon during the Apollo 17 mission are composed of 12.one% TiOii.[seven] Native titanium (pure metallic) is very rare.[26]
Isotopes
Naturally occurring titanium is composed of five stable isotopes: 46Ti, 47Ti, 48Ti, 49Ti, and 50Ti, with 48Ti beingness the nigh abundant (73.eight% natural abundance). At least 21 radioisotopes take been characterized, the most stable of which are 44Ti with a half-life of 63 years; 45Ti, 184.8 minutes; 51Ti, 5.76 minutes; and 52Ti, 1.7 minutes. All other radioactive isotopes have one-half-lives less than 33 seconds, with the majority less than half a 2nd.[12]
The isotopes of titanium range in atomic weight from 39.002 u (39Ti) to 63.999 u (64Ti).[27] The primary decay way for isotopes lighter than 46Ti is positron emission (with the exception of 44Ti which undergoes electron capture), leading to isotopes of scandium, and the main mode for isotopes heavier than 50Ti is beta emission, leading to isotopes of vanadium.[12]
Titanium becomes radioactive upon bombardment with deuterons, emitting mainly positrons and difficult gamma rays.[vii]
Compounds

The +4 oxidation state dominates titanium chemical science,[28] but compounds in the +3 oxidation country are also numerous.[29] Normally, titanium adopts an octahedral coordination geometry in its complexes,[thirty] [31] but tetrahedral TiCl4 is a notable exception. Because of its high oxidation country, titanium(IV) compounds exhibit a high degree of covalent bonding.[28]
Oxides, sulfides, and alkoxides
The most important oxide is TiO2, which exists in iii of import polymorphs; anatase, brookite, and rutile. All three are white diamagnetic solids, although mineral samples can appear dark (come across rutile). They adopt polymeric structures in which Ti is surrounded by six oxide ligands that link to other Ti centers.[32]
The term titanates usually refers to titanium(Four) compounds, as represented by barium titanate (BaTiO3). With a perovskite structure, this material exhibits piezoelectric backdrop and is used as a transducer in the interconversion of sound and electricity.[eleven] Many minerals are titanates, such as ilmenite (FeTiOiii). Star sapphires and rubies get their asterism (star-forming polish) from the presence of titanium dioxide impurities.[19]
A variety of reduced oxides (suboxides) of titanium are known, mainly reduced stoichiometries of titanium dioxide obtained by atmospheric plasma spraying. Ti3O5, described as a Ti(Iv)-Ti(III) species, is a purple semiconductor produced by reduction of TiO2 with hydrogen at high temperatures,[33] and is used industrially when surfaces demand to exist vapor-coated with titanium dioxide: it evaporates equally pure TiO, whereas TiO2 evaporates as a mixture of oxides and deposits coatings with variable refractive index.[34] Also known is TitwoOthree, with the corundum structure, and TiO, with the stone salt construction, although oft nonstoichiometric.[35]
The alkoxides of titanium(Four), prepared by treating TiCliv with alcohols, are colorless compounds that convert to the dioxide on reaction with water. They are industrially useful for depositing solid TiO2 via the sol-gel process. Titanium isopropoxide is used in the synthesis of chiral organic compounds via the Sharpless epoxidation.[36]
Titanium forms a variety of sulfides, but just TiS2 has attracted pregnant interest. It adopts a layered structure and was used as a cathode in the evolution of lithium batteries. Because Ti(IV) is a "difficult cation", the sulfides of titanium are unstable and tend to hydrolyze to the oxide with release of hydrogen sulfide.[37]
Nitrides and carbides
Titanium nitride (TiN) is a refractory solid exhibiting extreme hardness, thermal/electrical conductivity, and a high melting bespeak.[38] TiN has a hardness equivalent to sapphire and carborundum (9.0 on the Mohs scale),[39] and is frequently used to coat cutting tools, such equally drill bits.[40] It is also used as a gilt-colored decorative finish and as a bulwark layer in semiconductor fabrication.[41] Titanium carbide (TiC), which is also very difficult, is plant in cutting tools and coatings.[42]
Halides

Titanium(III) compounds are characteristically violet, illustrated by this aqueous solution of titanium trichloride.
Titanium tetrachloride (titanium(IV) chloride, TiCl4 [43]) is a colorless volatile liquid (commercial samples are yellowish) that, in air, hydrolyzes with spectacular emission of white clouds. Via the Kroll process, TiCl4 is used in the conversion of titanium ores to titanium metallic. Titanium tetrachloride is too used to make titanium dioxide, e.grand., for use in white pigment.[44] It is widely used in organic chemistry as a Lewis acid, for case in the Mukaiyama aldol condensation.[45] In the van Arkel–de Boer process, titanium tetraiodide (TiI4) is generated in the production of high purity titanium metal.[46]
Titanium(III) and titanium(2) also form stable chlorides. A notable example is titanium(3) chloride (TiCl3), which is used as a goad for product of polyolefins (see Ziegler–Natta catalyst) and a reducing agent in organic chemistry.[47]
Organometallic complexes
Owing to the important role of titanium compounds equally polymerization catalyst, compounds with Ti-C bonds have been intensively studied. The most mutual organotitanium circuitous is titanocene dichloride ((C5H5)2TiCl2). Related compounds include Tebbe's reagent and Petasis reagent. Titanium forms carbonyl complexes, e.m. (CfiveH5)2Ti(CO)ii.[48]
Anticancer therapy studies
Following the success of platinum-based chemotherapy, titanium(Four) complexes were among the beginning non-platinum compounds to be tested for cancer treatment. The reward of titanium compounds lies in their loftier efficacy and depression toxicity in vivo.[49] In biological environments, hydrolysis leads to the safe and inert titanium dioxide. Despite these advantages the first candidate compounds failed clinical trials due to insufficient efficacy to toxicity ratios and formulation complications.[49] Further development resulted in the creation of potentially effective, selective, and stable titanium-based drugs.[49]
History

Titanium was discovered in 1791 by the clergyman and amateur geologist William Gregor every bit an inclusion of a mineral in Cornwall, U.k..[50] Gregor recognized the presence of a new element in ilmenite[eight] when he found black sand by a stream and noticed the sand was attracted by a magnet.[l] Analyzing the sand, he determined the presence of two metal oxides: atomic number 26 oxide (explaining the attraction to the magnet) and 45.25% of a white metallic oxide he could not identify.[22] Realizing that the unidentified oxide independent a metal that did not lucifer whatsoever known element, Gregor reported his findings to the Purple Geological Society of Cornwall and in the German language science journal Crell's Annalen.[50] [51] [52]
Effectually the same time, Franz-Joseph Müller von Reichenstein produced a similar substance, but could not identify it.[eight] The oxide was independently rediscovered in 1795 past Prussian chemist Martin Heinrich Klaproth in rutile from Boinik (the German proper name of Bajmócska), a village in Hungary (at present Bojničky in Slovakia).[50] [53] Klaproth institute that it contained a new element and named information technology for the Titans of Greek mythology.[25] Afterwards hearing about Gregor's earlier discovery, he obtained a sample of manaccanite and confirmed that it contained titanium.[54]
The currently known processes for extracting titanium from its various ores are laborious and costly; it is not possible to reduce the ore by heating with carbon (as in iron smelting) because titanium combines with the carbon to produce titanium carbide.[50] Pure metallic titanium (99.9%) was start prepared in 1910 by Matthew A. Hunter at Rensselaer Polytechnic Establish past heating TiCl4 with sodium at 700–800 °C under great force per unit area[55] in a batch procedure known as the Hunter procedure.[7] Titanium metal was not used outside the laboratory until 1932 when William Justin Kroll produced it by reducing titanium tetrachloride (TiCl4) with calcium.[56] Eight years after he refined this process with magnesium and with sodium in what became known as the Kroll process.[56] Although research continues to seek cheaper and more than efficient routes, such as the FFC Cambridge process, the Kroll process is even so predominantly used for commercial production.[vii] [8]

Titanium of very high purity was fabricated in small quantities when Anton Eduard van Arkel and Jan Hendrik de Boer discovered the iodide process in 1925, past reacting with iodine and decomposing the formed vapors over a hot filament to pure metal.[57]
In the 1950s and 1960s, the Soviet Wedlock pioneered the use of titanium in military and submarine applications[55] (Alfa class and Mike class)[58] equally part of programs related to the Cold War.[59] Starting in the early 1950s, titanium came into use extensively in armed services aviation, particularly in high-operation jets, starting with aircraft such every bit the F-100 Super Sabre and Lockheed A-12 and SR-71.[lx]
Throughout the Cold War period, titanium was considered a strategic material by the U.S. government, and a large stockpile of titanium sponge (a porous grade of the pure metal) was maintained by the Defence force National Stockpile Center, until the stockpile was dispersed in the 2000s.[61] As of 2021, the four leading producers of titanium sponge were China (52%), Nippon (24%), Russia (16%) and Kazakhstan (vii%).[23]
Production

Titanium (mineral concentrate)

Basic titanium products: plate, tube, rods, and powder
The processing of titanium metal occurs in four major steps: reduction of titanium ore into "sponge", a porous class; melting of sponge, or sponge plus a master blend to form an ingot; primary fabrication, where an ingot is converted into general mill products such as billet, bar, plate, sheet, strip, and tube; and secondary fabrication of finished shapes from mill products.[62]
Because it cannot exist readily produced by reduction of titanium dioxide,[13] titanium metallic is obtained by reduction of TiCl4 with magnesium metallic in the Kroll procedure. The complexity of this batch production in the Kroll process explains the relatively high market value of titanium,[63] despite the Kroll process being less expensive than the Hunter process.[55] To produce the TiCl4 required by the Kroll process, the dioxide is subjected to carbothermic reduction in the presence of chlorine. In this process, the chlorine gas is passed over a cerise-hot mixture of rutile or ilmenite in the presence of carbon. After extensive purification by fractional distillation, the TiCl4 is reduced with 800 °C (i,470 °F) molten magnesium in an argon atmosphere.[xi] Titanium metal can be farther purified past the van Arkel–de Boer process, which involves thermal decomposition of titanium tetraiodide.
- 2 FeTiO3 + 7 Cl2 + 6 C → 2 TiClfour + ii FeCl3 + half-dozen CO (900 °C)
- TiClfour + two Mg → 2 MgCl2 + Ti (i,100 °C)
A more recently adult batch production method, the FFC Cambridge process,[64] reduces titanium dioxide electrochemically in molten calcium chloride to produce titanium metal as either powder or sponge.[65]
Mutual titanium alloys are made by reduction. For example, cuprotitanium (rutile with copper added is reduced), ferrocarbon titanium (ilmenite reduced with coke in an electric furnace), and manganotitanium (rutile with manganese or manganese oxides) are reduced.[66]
About l grades of titanium alloys are designed and currently used, although merely a couple of dozen are readily available commercially.[67] The ASTM International recognizes 31 grades of titanium metal and alloys, of which grades one through four are commercially pure (unalloyed). Those four vary in tensile strength equally a function of oxygen content, with course 1 being the nearly ductile (lowest tensile strength with an oxygen content of 0.18%), and grade 4 the least ductile (highest tensile strength with an oxygen content of 0.40%).[19] The remaining grades are alloys, each designed for specific properties of ductility, strength, hardness, electrical resistivity, pitter-patter resistance, specific corrosion resistance, and combinations thereof.[68]
In addition to the ASTM specifications, titanium alloys are likewise produced to come across aerospace and armed services specifications (SAE-AMS, MIL-T), ISO standards, and country-specific specifications, besides equally proprietary finish-user specifications for aerospace, armed forces, medical, and industrial applications.[69]
Titanium powder is manufactured using a flow production process known as the Armstrong procedure[70] that is similar to the batch production Hunter procedure. A stream of titanium tetrachloride gas is added to a stream of molten sodium; the products (sodium chloride salt and titanium particles) is filtered from the extra sodium. Titanium is then separated from the table salt by h2o washing. Both sodium and chlorine are recycled to produce and procedure more titanium tetrachloride.[71]
Fabrication
All welding of titanium must be done in an inert temper of argon or helium to shield it from contamination with atmospheric gases (oxygen, nitrogen, and hydrogen).[17] Contamination causes a variety of conditions, such as embrittlement, which reduce the integrity of the associates welds and atomic number 82 to articulation failure.[72]
Titanium is very difficult to solder straight, and hence a solderable metallic or alloy such as steel is coated on titanium prior to soldering.[73] Titanium metal tin be machined with the same equipment and the aforementioned processes as stainless steel.[17]
Forming and forging
Commercially pure apartment product (sheet, plate) tin exist formed readily, but processing must take into account of the tendency of the metal to springback. This is especially truthful of sure high-strength alloys.[74] [75] Exposure to the oxygen in air at the elevated temperatures used in forging results in formation of an brittle oxygen-rich metallic surface layer called "alpha case" that worsens the fatigue properties, so it must be removed by milling, etching, or electrochemical treatment.[76]
Applications

A titanium cylinder of "grade 2" quality
Titanium is used in steel as an alloying element (ferro-titanium) to reduce grain size and as a deoxidizer, and in stainless steel to reduce carbon content.[half-dozen] Titanium is often alloyed with aluminium (to refine grain size), vanadium, copper (to harden), iron, manganese, molybdenum, and other metals.[77] Titanium factory products (sheet, plate, bar, wire, forgings, castings) find application in industrial, aerospace, recreational, and emerging markets. Powdered titanium is used in pyrotechnics as a source of bright-burning particles.[78]
Pigments, additives, and coatings

About 95% of all titanium ore is destined for refinement into titanium dioxide (TiO
2 ), an intensely white permanent paint used in paints, newspaper, toothpaste, and plastics.[23] It is likewise used in cement, in gemstones, as an optical opacifier in paper,[79] and a strengthening agent in graphite composite fishing rods and golf game clubs.[80]
TiO
2 pigment is chemically inert, resists fading in sunlight, and is very opaque: it imparts a pure and brilliant white color to the brown or grey chemicals that class the majority of household plastics.[eight] In nature, this compound is found in the minerals anatase, brookite, and rutile.[6] Paint made with titanium dioxide does well in severe temperatures and marine environments.[8] Pure titanium dioxide has a very loftier index of refraction and an optical dispersion higher than diamond.[7] In addition to being a very important pigment, titanium dioxide is also used in sunscreens.[13]
Aerospace and marine
Considering titanium alloys have high tensile force to density ratio,[11] high corrosion resistance,[7] fatigue resistance, high crack resistance,[81] and ability to withstand moderately high temperatures without creeping, they are used in aircraft, armor plating, naval ships, spacecraft, and missiles.[seven] [8] For these applications, titanium is alloyed with aluminium, zirconium, nickel,[82] vanadium, and other elements to manufacture a variety of components including critical structural parts, fire walls, landing gear, exhaust ducts (helicopters), and hydraulic systems. In fact, most ii thirds of all titanium metal produced is used in aircraft engines and frames.[83] The titanium 6AL-4V blend accounts for almost 50% of all alloys used in aircraft applications.[84]
The Lockheed A-12 and its development the SR-71 "Blackbird" were ii of the first aircraft frames where titanium was used, paving the way for much wider use in mod armed services and commercial shipping. An estimated 116 metric tons are used in the Boeing 787, 77 in the Airbus A380, 59 in the Boeing 777, 45 in the Boeing 747, 18 in the Boeing 737, 32 in the Airbus A340, 18 in the Airbus A330, and 12 in the Airbus A320.[85] In aero engine applications, titanium is used for rotors, compressor blades, hydraulic organisation components, and nacelles.[ citation needed ] An early use in jet engines was for the Orenda Iroquois in the 1950s.[ better source needed ] [86]
Considering titanium is resistant to corrosion by sea water, it is used to make propeller shafts, rigging, and heat exchangers in desalination plants;[vii] heater-chillers for salt water aquariums, fishing line and leader, and defined' knives. Titanium is used in the housings and components of sea-deployed surveillance and monitoring devices for science and the military. The former Soviet Union developed techniques for making submarines with hulls of titanium alloys[87] forging titanium in huge vacuum tubes.[82]
Titanium is used in the walls of the Juno spacecraft'south vault to shield on-board electronics.[88]
Industrial

Welded titanium piping and process equipment (heat exchangers, tanks, process vessels, valves) are used in the chemical and petrochemical industries primarily for corrosion resistance. Specific alloys are used in oil and gas downhole applications and nickel hydrometallurgy for their high strength (e. k.: titanium beta C alloy), corrosion resistance, or both. The pulp and newspaper industry uses titanium in procedure equipment exposed to corrosive media, such as sodium hypochlorite or wet chlorine gas (in the bleachery).[89] Other applications include ultrasonic welding, wave soldering,[90] and sputtering targets.[91]
Titanium tetrachloride (TiCl4), a colorless liquid, is important equally an intermediate in the process of making TiO2 and is also used to produce the Ziegler–Natta goad. Titanium tetrachloride is also used to iridize glass and, because it fumes strongly in moist air, it is used to make smoke screens.[thirteen]
Consumer and architectural
Titanium metal is used in automotive applications, particularly in automobile and motorcycle racing where low weight and high strength and rigidity are critical.[92] The metal is more often than not also expensive for the general consumer market, though some late model Corvettes accept been manufactured with titanium exhausts,[93] and a Corvette Z06's LT4 supercharged engine uses lightweight, solid titanium intake valves for greater strength and resistance to oestrus.[94]
Titanium is used in many sporting goods: tennis rackets, golf clubs, lacrosse stick shafts; cricket, hockey, lacrosse, and football helmet grills, and bike frames and components. Although non a mainstream material for cycle production, titanium bikes have been used past racing teams and run a risk cyclists.[95]
Titanium alloys are used in spectacle frames that are rather expensive only highly durable, long lasting, light weight, and cause no skin allergies. Many backpackers use titanium equipment, including cookware, eating utensils, lanterns, and tent stakes. Though slightly more expensive than traditional steel or aluminium alternatives, titanium products tin be significantly lighter without compromising strength. Titanium horseshoes are preferred to steel by farriers considering they are lighter and more durable.[96]

Titanium has occasionally been used in architecture. The 42.5 chiliad (139 ft) Monument to Yuri Gagarin, the first man to travel in space ( 55°42′29.vii″N 37°34′57.2″E / 55.708250°N 37.582556°Due east / 55.708250; 37.582556 ), besides as the 110 m (360 ft) Monument to the Conquerors of Space on top of the Cosmonaut Museum in Moscow are made of titanium for the metal'southward attractive color and association with rocketry.[97] [98] The Guggenheim Museum Bilbao and the Cerritos Millennium Library were the first buildings in Europe and North America, respectively, to be sheathed in titanium panels.[83] Titanium sheathing was used in the Frederic C. Hamilton Building in Denver, Colorado.[99]
Because of titanium'south superior strength and low-cal weight relative to other metals (steel, stainless steel, and aluminium), and because of contempo advances in metalworking techniques, its use has go more widespread in the manufacture of firearms. Main uses include pistol frames and revolver cylinders. For the same reasons, information technology is used in the body of laptop computers (for instance, in Apple's PowerBook line).[100] [101]
Some upmarket lightweight and corrosion-resistant tools, such equally shovels, knife handles and flashlights, are made of titanium or titanium alloys.[101]
Jewelry
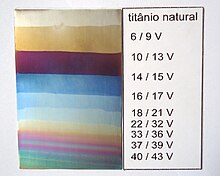
Relation between voltage and colour for anodized titanium
Because of its durability, titanium has go more than popular for designer jewelry (particularly, titanium rings).[96] Its inertness makes it a expert pick for those with allergies or those who will be wearing the jewelry in environments such as swimming pools. Titanium is also alloyed with gilded to produce an blend that can exist marketed as 24-karat gold because the ane% of alloyed Ti is insufficient to require a lesser mark. The resulting blend is roughly the hardness of 14-karat gilt and is more durable than pure 24-karat gold.[102]
Titanium'southward durability, calorie-free weight, and dent and corrosion resistance go far useful for watch cases.[96] Some artists work with titanium to produce sculptures, decorative objects and furniture.[103]
Titanium may be anodized to vary the thickness of the surface oxide layer, causing optical interference fringes and a variety of brilliant colors.[104] With this coloration and chemic inertness, titanium is a popular metallic for body piercing.[105]
Titanium has a minor employ in dedicated non-circulating coins and medals. In 1999, Gibraltar released the world's first titanium coin for the millennium celebration.[106] The Gilt Declension Titans, an Australian rugby league team, accolade a medal of pure titanium to their thespian of the yr.[107]
Medical
Because titanium is biocompatible (not-toxic and non rejected by the body), information technology has many medical uses, including surgical implements and implants, such as hip balls and sockets (joint replacement) and dental implants that can stay in place for up to 20 years.[fifty] The titanium is often alloyed with about 4% aluminium or 6% Al and 4% vanadium.[108]

Medical screws and plate used to repair wrist fractures. Scale is in centimeters.
Titanium has the inherent power to osseointegrate, enabling apply in dental implants that can last for over thirty years. This belongings is also useful for orthopedic implant applications.[50] These benefit from titanium's lower modulus of elasticity (Young's modulus) to more closely friction match that of the os that such devices are intended to repair. Equally a result, skeletal loads are more evenly shared between bone and implant, leading to a lower incidence of bone deposition due to stress shielding and periprosthetic bone fractures, which occur at the boundaries of orthopedic implants. Yet, titanium alloys' stiffness is still more than twice that of bone, then adjacent os bears a greatly reduced load and may deteriorate.[109] [110]
Because titanium is not-ferromagnetic, patients with titanium implants can be safely examined with magnetic resonance imaging (convenient for long-term implants). Preparing titanium for implantation in the body involves subjecting it to a loftier-temperature plasma arc which removes the surface atoms, exposing fresh titanium that is instantly oxidized.[50]
Mod advancements in additive manufacturing techniques accept increased potential for titanium use in orthopedic implant applications.[111] Complex implant scaffold designs tin be 3D-printed using titanium alloys, which allows for more patient-specific applications and increased implant osseointegration.[112]
Titanium is used for the surgical instruments used in image-guided surgery, as well equally wheelchairs, crutches, and any other products where high strength and depression weight are desirable.[113]
Titanium dioxide nanoparticles are widely used in electronics and the commitment of pharmaceuticals and cosmetics.[114]
Nuclear waste storage
Because of its corrosion resistance, containers fabricated of titanium have been studied for the long-term storage of nuclear waste. Containers lasting more 100,000 years are thought possible with manufacturing conditions that minimize textile defects.[115] A titanium "drip shield" could too be installed over containers of other types to raise their longevity.[116]
Precautions
Titanium is non-toxic fifty-fifty in large doses and does not play whatever natural role inside the human trunk.[25] An estimated quantity of 0.8 milligrams of titanium is ingested by humans each day, simply well-nigh passes through without being absorbed in the tissues.[25] It does, still, sometimes bio-accumulate in tissues that comprise silica. One study indicates a possible connection between titanium and yellow blast syndrome.[117]
As a pulverisation or in the grade of metallic shavings, titanium metal poses a pregnant burn adventure and, when heated in air, an explosion hazard.[118] Water and carbon dioxide are ineffective for extinguishing a titanium burn; Class D dry powder agents must be used instead.[8]
When used in the production or handling of chlorine, titanium should not be exposed to dry chlorine gas because it may result in a titanium–chlorine fire.[119]
Titanium can take hold of burn down when a fresh, non-oxidized surface comes in contact with liquid oxygen.[120]
Role in plants

Nettles contain upward to eighty parts per million of titanium.[25]
An unknown mechanism in plants may use titanium to stimulate the production of carbohydrates and encourage growth. This may explain why virtually plants contain nearly 1 function per 1000000 (ppm) of titanium, food plants have nearly 2 ppm, and horsetail and nettle contain upward to eighty ppm.[25]
See also
- List of countries by titanium product
- Suboxide
- Titanium in Africa
- Titanium in zircon geothermometry
- Titanium Man
- VSMPO-AVISMA
References
- ^ "titanium". Oxford Dictionaries UK English Lexicon. Oxford University Press. n.d. Retrieved 28 March 2017.
- ^ "Standard Atomic Weights: Titanium". CIAAW. 1993.
- ^ Jilek, Robert E.; Tripepi, Giovanna; Urnezius, Eugenijus; Brennessel, William W.; Young, Victor G., Jr.; Ellis, John E. (2007). "Zerovalent titanium–sulfur complexes. Novel dithiocarbamato derivatives of Ti(CO)6: [Ti(CO)iv(SiiCNRtwo)]−". Chem. Commun. (25): 2639–2641. doi:x.1039/B700808B. PMID 17579764.
- ^ Andersson, North.; et al. (2003). "Emission spectra of TiH and TiD nearly 938 nm" (PDF). J. Chem. Phys. 118 (8): 10543. Bibcode:2003JChPh.118.3543A. doi:10.1063/1.1539848.
- ^ Weast, Robert (1984). CRC, Handbook of Chemical science and Physics. Boca Raton, Florida: Chemical Rubber Company Publishing. pp. E110. ISBN0-8493-0464-4.
- ^ a b c d e f g h i "Titanium". Encyclopædia Britannica. 2006. Retrieved xix January 2022.
- ^ a b c d e f 1000 h i j thousand 50 m Lide, D. R., ed. (2005). CRC Handbook of Chemical science and Physics (86th ed.). Boca Raton (FL): CRC Printing. ISBN0-8493-0486-v.
- ^ a b c d e f g h i Krebs, Robert E. (2006). The History and Use of Our Globe's Chemical Elements: A Reference Guide (2nd ed.). Westport, CT: Greenwood Press. ISBN978-0-313-33438-two.
- ^ Donachie 1988, p. xi
- ^ a b Barksdale 1968, p. 738
- ^ a b c d e f "Titanium". Columbia Encyclopedia (6th ed.). New York: Columbia University Press. 2000–2006. ISBN978-0-7876-5015-5.
- ^ a b c Barbalace, Kenneth 50. (2006). "Periodic Table of Elements: Ti – Titanium". Retrieved 26 Dec 2006.
- ^ a b c d e Stwertka, Albert (1998). "Titanium". Guide to the Elements (Revised ed.). Oxford University Printing. pp. 81–82. ISBN978-0-19-508083-4.
- ^ Steele, Thou. C.; Hein, R. A. (1953). "Superconductivity of Titanium". Phys. Rev. 92 (two): 243–247. Bibcode:1953PhRv...92..243S. doi:10.1103/PhysRev.92.243.
- ^ Thiemann, Chiliad.; et al. (2018). "Consummate electrodynamics of a BCS superconductor with μeV energy scales: Microwave spectroscopy on titanium at mK temperatures". Phys. Rev. B. 97 (21): 214516. arXiv:1803.02736. Bibcode:2018PhRvB..97u4516T. doi:10.1103/PhysRevB.97.214516. S2CID 54891002.
- ^ Donachie 1988, Appendix J, Table J.2
- ^ a b c d eastward Barksdale 1968, p. 734
- ^ Puigdomenech, Ignasi (2004) Hydra/Medusa Chemical Equilibrium Database and Plotting Software, KTH Royal Constitute of Applied science.
- ^ a b c d eastward Emsley 2001, p. 453
- ^ Casillas, N.; Charlebois, S.; Smyrl, Due west. H.; White, H. South. (1994). "Pitting Corrosion of Titanium" (PDF). J. Electrochem. Soc. 141 (3): 636–642. Bibcode:1994JElS..141..636C. doi:10.1149/1.2054783. Archived from the original (PDF) on 27 August 2020.
- ^ Forrest, A. Fifty. (1981). "Furnishings of Metallic Chemistry on Behavior of Titanium in Industrial Applications". Industrial Applications of Titanium and Zirconium. p. 112.
- ^ a b c Barksdale 1968, p. 732
- ^ a b c d e The states Geological Survey. "USGS Minerals Information: Titanium".
- ^ Buettner, One thousand. M.; Valentine, A. M. (2012). "Bioinorganic Chemical science of Titanium". Chemic Reviews. 112 (3): 1863–81. doi:10.1021/cr1002886. PMID 22074443.
- ^ a b c d east f Emsley 2001, p. 451
- ^ Titanium. Mindat
- ^ Wang, Grand.; Audi, G.; Kondev, F. G.; Huang, Westward. J.; Naimi, S.; Xu, Ten. (2017). "The AME2016 atomic mass evaluation (Ii). Tables, graphs, and references" (PDF). Chinese Physics C. 41 (three): 030003-one–030003-442. doi:10.1088/1674-1137/41/3/030003.
- ^ a b Greenwood & Earnshaw 1997, p. 958
- ^ Greenwood & Earnshaw 1997, p. 970
- ^ Greenwood & Earnshaw 1997, p. 960
- ^ Greenwood & Earnshaw 1997, p. 967
- ^ Greenwood & Earnshaw 1997, p. 961
- ^ Liu, Gang; Huang, Wan-Xia; Yi, Yong (26 June 2013). "Preparation and Optical Storage Properties of λTi3Ofive Powder". Journal of Inorganic Materials. 28 (4): 425–430. doi:10.3724/SP.J.1077.2013.12309.
- ^ Bonardi, Antonio; Pühlhofer, Gerd; Hermanutz, Stephan; Santangelo, Andrea (2014). "A new solution for mirror coating in $γ$-ray Cherenkov Astronomy". Experimental Astronomy. 38 (1–2): 1–ix. arXiv:1406.0622. Bibcode:2014ExA....38....1B. doi:x.1007/s10686-014-9398-x. S2CID 119213226.
- ^ Greenwood & Earnshaw 1997, p. 962.
- ^ Ramón, Diego J.; Yus, Miguel (2006). "In the Arena of Enantioselective Synthesis, Titanium Complexes Wear the Laurel Wreath". Chem. Rev. 106 (6): 2126–2308. doi:ten.1021/cr040698p. PMID 16771446.
- ^ McKelvy, M. J.; Glaunsinger, W. S. (1995). "Titanium Disulfide". Inorganic Syntheses. Inorganic Syntheses. Vol. xxx. pp. 28–32. doi:10.1002/9780470132616.ch7. ISBN9780470132616.
- ^ Saha, Naresh (1992). "Titanium nitride oxidation chemistry: An x-ray photoelectron spectroscopy written report". Journal of Applied Physics. 72 (7): 3072–3079. Bibcode:1992JAP....72.3072S. doi:10.1063/one.351465.
- ^ Schubert, E.F. "The hardness calibration introduced by Friederich Mohs" (PDF). Archived (PDF) from the original on 3 June 2010.
- ^ Truini, Joseph (May 1988). "Drill Bits". Popular Mechanics. 165 (5): 91. ISSN 0032-4558.
- ^ Baliga, B. Jayant (2005). Silicon carbide ability devices. World Scientific. p. 91. ISBN978-981-256-605-8.
- ^ "Titanium carbide product information". H. C. Starck. Archived from the original on 22 September 2017. Retrieved 16 November 2015.
- ^ Seong, S.; et al. (2009). Titanium: industrial base, cost trends, and technology initiatives. Rand Corporation. p. 10. ISBN978-0-8330-4575-i.
- ^ Johnson, Richard W. (1998). The Handbook of Fluid Dynamics. Springer. pp. 38–21. ISBN978-iii-540-64612-9.
- ^ Coates, Robert M.; Paquette, Leo A. (2000). Handbook of Reagents for Organic Synthesis. John Wiley and Sons. p. 93. ISBN978-0-470-85625-3.
- ^ Greenwood & Earnshaw 1997, p. 965
- ^ Gundersen, Lise-Lotte; Rise, Frode; Undheim, Kjell; Méndez Andino, José (2007). "Titanium(Iii) Chloride". Encyclopedia of Reagents for Organic Synthesis. doi:10.1002/047084289X.rt120.pub2. ISBN978-0471936237.
- ^ Hartwig, J. F. (2010) Organotransition Metal Chemistry, from Bonding to Catalysis. Academy Scientific discipline Books: New York. ISBN 189138953X
- ^ a b c Tshuva, Edit Y.; Miller, Maya (2018). "Chapter 8. Coordination Complexes of Titanium(IV) for Anticancer Therapy". In Sigel, Astrid; Sigel, Helmut; Freisinger, Eva; Sigel, Roland M. O. (eds.). Metallo-Drugs: Development and Action of Anticancer Agents. Metallic Ions in Life Sciences. Vol. eighteen. Berlin: de Gruyter GmbH. pp. 219–250. doi:x.1515/9783110470734-014. ISBN9783110470734. PMID 29394027.
- ^ a b c d e f 1000 h Emsley 2001, p. 452
- ^ Gregor, William (1791) "Beobachtungen und Versuche über den Menakanit, einen in Cornwall gefundenen magnetischen Sand" (Observations and experiments regarding menaccanite [i.eastward., ilmenite], a magnetic sand constitute in Cornwall), Chemische Annalen …, 1, pp. 40–54, 103–119.
- ^ Gregor, William (1791) "Sur le menakanite, espèce de sable attirable par l'aimant, trouvé dans la province de Cornouilles" (On menaccanite, a species of magnetic sand, found in the county of Cornwall), Observations et Mémoires sur la Physique, 39: 72–78, 152–160.
- ^ Klaproth, Martin Heinrich (1795) "Chemische Untersuchung des sogenannten hungarischen rothen Schörls" (Chemical investigation of the then-called Hungarian reddish tourmaline [rutile]) in: Beiträge zur chemischen Kenntniss der Mineralkörper (Contributions to the chemical noesis of mineral substances), vol. 1, (Berlin, Germany): Heinrich August Rottmann, 233–244. From page 244: "Diesem zufolge will ich den Namen für die gegenwärtige metallische Substanz, gleichergestalt wie bei dem Uranium geschehen, aus der Mythologie, und zwar von den Ursöhnen der Erde, den Titanen, entlehnen, und benenne also diese neue Metallgeschlecht: Titanium; … " (By virtue of this I will derive the name for the nowadays metallic substance — as happened similarly in the case of uranium — from mythology, namely from the first sons of the Earth, the Titans, and thus [I] name this new species of metal: "titanium"; … )
- ^ Suisman Titanium Corporation (1995). Twenty-five Years of Titanium News. Pennsylvania State University. p. 37.
- ^ a b c Roza 2008, p. nine
- ^ a b Greenwood & Earnshaw 1997, p. 955
- ^ van Arkel, A. Due east.; de Boer, J. H. (1925). "Preparation of pure titanium, zirconium, hafnium, and thorium metal". Zeitschrift für anorganische und allgemeine Chemie. 148: 345–l. doi:x.1002/zaac.19251480133.
- ^ Yanko, Eugene (2006). "Submarines: general information". Omsk VTTV Arms Exhibition and Armed forces Parade JSC. Archived from the original on 6 April 2016. Retrieved two Feb 2015.
- ^ Stainless Steel World (July–August 2001). "VSMPO Stronger Than E'er" (PDF). KCI Publishing B.V. pp. 16–19. Archived from the original (PDF) on 5 October 2006. Retrieved two January 2007.
- ^ Jasper, Adam, ed. (2020). Architecture and Anthropology. Taylor & Francis. p. 42. ISBN9781351106276.
- ^ Defense National Stockpile Centre (2008). Strategic and Critical Materials Report to the Congress. Operations under the Strategic and Critical Materials Stock Piling Act during the Period October 2007 through September 2008 (PDF). U.s.a. Section of Defence force. p. 3304. Archived from the original (PDF) on 11 February 2010.
- ^ Donachie 1988, Ch. four
- ^ Barksdale 1968, p. 733
- ^ Chen, George Zheng; Fray, Derek J.; Farthing, Tom W. (2000). "Direct electrochemical reduction of titanium dioxide to titanium in molten calcium chloride". Nature. 407 (6802): 361–364. Bibcode:2000Natur.407..361C. doi:10.1038/35030069. PMID 11014188. S2CID 205008890.
- ^ Roza 2008, p. 23
- ^ "Titanium". Microsoft Encarta. 2005. Archived from the original on 27 October 2006. Retrieved 29 December 2006.
- ^ Donachie 1988, p. 16, Appendix J
- ^ ASTM International (2006). Annual Book of ASTM Standards (Volume 02.04: Non-ferrous Metals). Due west Conshohocken, PA: ASTM International. department 2. ISBN978-0-8031-4086-8. ASTM International (1998). Annual Book of ASTM Standards (Book thirteen.01: Medical Devices; Emergency Medical Services). West Conshohocken, PA: ASTM International. sections 2 & 13. ISBN978-0-8031-2452-3.
- ^ Donachie 1988, pp. 13–16, Appendices H and J
- ^ Roza 2008, p. 25
- ^ "Titanium". The Essential Chemical Manufacture online. York, UK: CIEC Promoting Science at the Academy of York. 15 Jan 2015.
- ^ Engel, Abraham 50.; Huber, R. West.; Lane, I. R. (1955). Arc-welding Titanium. U.S. Section of the Interior, Bureau of Mines.
- ^ Lewis, West. J.; Faulkner, G. E.; Rieppel, P. J. (1956). Report on Brazing and Soldering of Titanium. Titanium Metallurgical Laboratory, Battelle Memorial Institute.
- ^ AWS G2.4/G2.4M:2007 Guide for the Fusion Welding of Titanium and Titanium Alloys. Miami: American Welding Order. 2006. Archived from the original on 10 December 2010.
{{cite book}}: CS1 maint: bot: original URL status unknown (link) - ^ Titanium Metals Corporation (1997). Titanium design and fabrication handbook for industrial applications. Dallas: Titanium Metals Corporation. Archived from the original on ix Feb 2009.
{{cite book}}: CS1 maint: bot: original URL status unknown (link) - ^ Chen, George Z.; Fray, Derek J.; Farthing, Tom Due west. (2001). "Cathodic deoxygenation of the alpha example on titanium and alloys in molten calcium chloride". Metall. Mater. Trans. B. 32 (six): 1041. doi:ten.1007/s11663-001-0093-eight. S2CID 95616531.
- ^ Hampel, Clifford A. (1968). The Encyclopedia of the Chemical Elements. Van Nostrand Reinhold. p. 738. ISBN978-0-442-15598-8.
- ^ Mocella, Chris; Conkling, John A. (2019). Chemistry of Pyrotechnics. CRC Press. p. 86. ISBN9781351626569.
- ^ Smook, Gary A. (2002). Handbook for Pulp & Paper Technologists (third ed.). Angus Wilde Publications. p. 223. ISBN978-0-9694628-five-ix.
- ^ University of Leeds (13 February 2008). "Cheap, Environmentally Friendly Extraction Method For Titanium Dioxide Adult". ScienceDaily . Retrieved 12 Nov 2021.
- ^ Moiseyev, Valentin North. (2006). Titanium Alloys: Russian Aircraft and Aerospace Applications. Taylor and Francis, LLC. p. 196. ISBN978-0-8493-3273-9.
- ^ a b Kramer, Andrew Due east. (5 July 2013). "Titanium Fills Vital Role for Boeing and Russia". The New York Times . Retrieved half-dozen July 2013.
- ^ a b Emsley 2001, p. 454
- ^ Donachie 1988, p. 13
- ^ Froes, F. H., ed. (2015). Titanium Physical Metallurgy, Processing, and Applications. ASM International. p. vii. ISBN9781627080804.
- ^ "Iroquois". Flight Annal. 1957. p. 412. Archived from the original on 13 Dec 2009.
- ^ "GlobalSecurity". GlobalSecurity.org. April 2006. Retrieved 23 April 2008.
- ^ Scharf, Caleb A. (17 June 2016) The Jupiter Vault. Scientific American.
- ^ Donachie 1988, pp. 11–sixteen
- ^ Kleefisch, E.Due west., ed. (1981). Industrial Awarding of Titanium and Zirconium. West Conshohocken, PA: ASTM International. ISBN978-0-8031-0745-8.
- ^ Bunshah, Rointan F., ed. (2001). "Ch. eight". Handbook of Hard Coatings. Norwich, NY: William Andrew Inc. ISBN978-0-8155-1438-one.
- ^ Bell, Tom; et al. (2001). Rut Treating. Proceedings of the 20th Conference, 9–12 October 2000. ASM International. p. 141. ISBN978-0-87170-727-seven.
- ^ National Corvette Museum (2006). "Titanium Exhausts". Archived from the original on 3 January 2013. Retrieved 26 December 2006.
- ^ Compact Powerhouse: Within Corvette Z06'south LT4 Engine 650-hp supercharged 6.2L V-8 makes world-grade power in more than efficient package. media.gm.com. 20 August 2014
- ^ Davis, Joseph R. (1998). Metals Handbook . ASM International. p. 584. ISBN978-0-87170-654-6.
- ^ a b c Donachie 1988, pp. xi, 255
- ^ Mike Gruntman (2004). Blazing the Trail: The Early on History of Spacecraft and Rocketry. Reston, VA: American Constitute of Helmsmanship and Astronautics. p. 457. ISBN978-1-56347-705-viii.
- ^ Lütjering, Gerd; Williams, James Instance (12 June 2007). "Appearance Related Applications". Titanium. ISBN978-3-540-71397-v.
- ^ "Denver Art Museum, Frederic C. Hamilton Building". SPG Media. 2006. Retrieved 26 December 2006.
- ^ "Apple PowerBook G4 400 (Original – Ti) Specs". everymac.com . Retrieved 8 August 2009.
- ^ a b Qian, Ma; Niinomi, Mitsuo (2019). Real-Globe Employ of Titanium. Elsevier Science. pp. 7–8. ISBN9780128158203.
- ^ Gafner, Yard. (1989). "The development of 990 Gold-Titanium: its Product, utilise and Backdrop" (PDF). Gilt Message. 22 (4): 112–122. doi:x.1007/BF03214709. S2CID 114336550. Archived from the original on 29 November 2010.
{{cite journal}}: CS1 maint: unfit URL (link) - ^ "Fine Art and Functional Works in Titanium and Other Earth Elements". Archived from the original on thirteen May 2008. Retrieved 8 August 2009.
{{cite spider web}}: CS1 maint: bot: original URL status unknown (link) - ^ Alwitt, Robert Southward. (2002). "Electrochemistry Encyclopedia". Chemical Engineering Department, Example Western Reserve University, U.S. Archived from the original on 2 July 2008. Retrieved xxx December 2006.
{{cite web}}: CS1 maint: unfit URL (link) - ^ "Body Piercing Safety". doctorgoodskin.com. i Baronial 2006.
- ^ "Globe Firsts". British Pobjoy Mint. Retrieved eleven November 2017.
- ^ Turgeon, Luke (20 September 2007). "Titanium Titan: Broughton immortalised". The Gilded Declension Bulletin. Archived from the original on 28 September 2013.
{{cite news}}: CS1 maint: unfit URL (link) - ^ "Orthopaedic Metallic Alloys". Totaljoints.info. Retrieved 27 September 2010.
- ^ "Titanium foams replace injured bones". Research News. one September 2010. Archived from the original on 4 September 2010. Retrieved 27 September 2010.
- ^ Lavine, Marc Southward. (11 Jan 2018). Vignieri, Sacha; Smith, Jesse (eds.). "Brand no bones near titanium". Science. 359 (6372): 173.six–174. Bibcode:2018Sci...359..173L. doi:10.1126/science.359.6372.173-f.
- ^ Harun, W.S.West.; Manam, North.S.; Kamariah, Grand.S.I.Northward.; Sharif, Southward.; Zulkifly, A.H.; Ahmad, I.; Miura, H. (2018). "A review of powdered additive manufacturing techniques for Ti-6al-4v biomedical applications" (PDF). Powder Technology. 331: 74–97. doi:ten.1016/j.powtec.2018.03.010.
- ^ Trevisan, Francesco; Calignano, Flaviana; Aversa, Alberta; Marchese, Giulio; Lombardi, Mariangela; Biamino, Sara; Ugues, Daniele; Manfredi, Diego (2017). "Additive manufacturing of titanium alloys in the biomedical field: processes, properties and applications". Journal of Applied Biomaterials & Functional Materials. 16 (2): 57–67. doi:ten.5301/jabfm.5000371. PMID 28967051. S2CID 27827821.
- ^ Qian, Ma; Niinomi, Mitsuo (2019). Real-World Employ of Titanium. Elsevier Science. pp. 51, 128. ISBN9780128158203.
- ^ Pinsino, Annalisa; Russo, Roberta; Bonaventura, Rosa; Brunelli, Andrea; Marcomini, Antonio; Matranga, Valeria (28 September 2015). "Titanium dioxide nanoparticles stimulate sea urchin allowed jail cell phagocytic activity involving TLR/p38 MAPK-mediated signalling pathway". Scientific Reports. five: 14492. Bibcode:2015NatSR...514492P. doi:ten.1038/srep14492. PMC4585977. PMID 26412401.
- ^ Shoesmith, D. Due west.; Noel, J. J.; Hardie, D.; Ikeda, B. M. (2000). "Hydrogen Absorption and the Lifetime Functioning of Titanium Nuclear Waste Containers". Corrosion Reviews. 18 (4–5): 331–360. doi:ten.1515/CORRREV.2000.eighteen.4-v.331. S2CID 137825823.
- ^ Carter, L. J.; Pigford, T. J. (2005). "Proof of Safe at Yucca Mountain". Science. 310 (5747): 447–8. doi:10.1126/scientific discipline.1112786. PMID 16239463. S2CID 128447596.
- ^ Berglund, Fredrik; Carlmark, Bjorn (October 2011). "Titanium, Sinusitis, and the Yellow Nail Syndrome". Biological Trace Element Inquiry. 143 (i): ane–7. doi:10.1007/s12011-010-8828-5. PMC3176400. PMID 20809268.
- ^ Cotell, Catherine Mary; Sprague, J. A.; Smidt, F. A. (1994). ASM Handbook: Surface Engineering science (tenth ed.). ASM International. p. 836. ISBN978-0-87170-384-2.
- ^ Compressed Gas Association (1999). Handbook of compressed gases (4th ed.). Springer. p. 323. ISBN978-0-412-78230-5.
- ^ Solomon, Robert E. (2002). Burn and Life Safe Inspection Manual. National Burn Prevention Association (eighth ed.). Jones & Bartlett Publishers. p. 45. ISBN978-0-87765-472-eight.
Bibliography
- Barksdale, Jelks (1968). "Titanium". In Clifford A. Hampel (ed.). The Encyclopedia of the Chemical Elements. New York: Reinhold Book Corporation. pp. 732–738. LCCN 68029938.
- Donachie, Matthew J., Jr. (1988). TITANIUM: A Technical Guide. Metals Park, OH: ASM International. p. eleven. ISBN978-0-87170-309-v.
- Emsley, John (2001). "Titanium". Nature's Building Blocks: An A-Z Guide to the Elements . Oxford, England, United kingdom: Oxford University Printing. ISBN978-0-19-850340-viii.
- Flower, Harvey M. (2000). "Materials Scientific discipline: A moving oxygen story". Nature. 407 (6802): 305–306. doi:x.1038/35030266. PMID 11014169.
- Greenwood, North. N.; Earnshaw, A. (1997). Chemical science of the Elements (2nd ed.). Oxford: Butterworth-Heinemann. ISBN978-0-7506-3365-9.
- Roza, Greg (2008). Titanium (Get-go ed.). New York, NY: The Rosen Publishing Group. ISBN978-1-4042-1412-5.
External links
- "Titanium: Our Next Major Metal", Pop Science, October 1950—one of outset full general public detailed articles on Titanium
- Titanium at The Periodic Table of Videos (University of Nottingham)
- Titanium at The Essential Chemical Industry – online (CIEC Promoting Science at the University of York)
- International Titanium Association Archived 4 Nov 2020 at the Wayback Machine
- Metallurgy of Titanium and its Alloys, Cambridge University
- World Product of Titanium Concentrates, past State
- Metal of the gods
Source: https://en.wikipedia.org/wiki/Titanium
Post a Comment for "What Family Is Titanium in on the Periodic Table"